What is an electric battery?
What is an electric battery?
 What is an electric battery?
What is an electric battery?
What is an electric battery? Electric batteries are used in many different applications, including solar energy systems.
It is used as a third source after solar panels and the electricity grid.
With the great popular demand for batteries, it can be said that electric batteries have become an essential element in our lives that we cannot do without at all.
The electric battery is used to operate cars, generators, solar energy systems, emergency systems, remotes, watches, and countless other important uses.
What is an electric battery
The battery was discovered during the fourteenth century by the Italian scientist “Volta”,
It consists of two electrodes of two different electrically conductive materials, immersed in an electrolyte solution.
One pole is positive and the other is negative, depending on the charge that each one carries.
The potential difference between the two electrodes depends on the type of material the electrodes are made of, as well as the type of electrolyte solution.
Batteries are mainly used to store electrical energy safely for use at any time and as needed.
Battery technology has now developed and diversified according to use, size and components.
The battery industry is still one of the most important sciences in research and study due to the importance of energy storage in developing many engineering and technological sectors.
Electric batteries
Primary batteries and secondary batteries. In terms of chemistry, they cannot be limited to one type. In terms of size, they have many types and various sizes, such as:
(AA ، AAA ، C ، F ، E ، D).
Electric battery sizes
In terms of chargeability, it is divided into two types:
Non-rechargeable primary batteries, and rechargeable secondary batteries.
Different shapes of rechargeable batteries
Solar batteries and car batteries
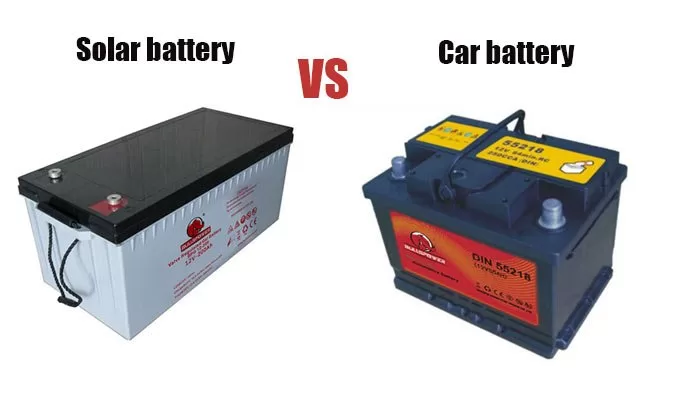 Solar batteries and car batteries
Solar batteries and car batteries
Solar batteries, car batteries,
Some may think that car batteries are suitable for solar energy systems, just like deep cycle batteries.
There is no impact on the installation of batteries intended for cars, and these beliefs are wrong.
Because there is a big difference between the two types in terms of composition and function, and each type has its own field.
Car batteries
 They are batteries intended for cars only because they are designed to give a high starting current to ignite the car engine.
They are batteries intended for cars only because they are designed to give a high starting current to ignite the car engine.
The main reason why it is not suitable for solar energy systems is that the number of charging and discharging cycles is very small and it is not of the deep cycle type.
Some may resort to installing car batteries for the solar system because of their cheap price compared to solar batteries.
But if you take into consideration the lifespan of the battery and the number of charging and discharging cycles it has, you will notice that deep cycle batteries are better and less expensive than a car battery in the long run.
Another reason is the lack of study of the difference between the specifications of solar batteries and car batteries and the advantages of deep cycle batteries over car batteries.
Solar batteries
 These batteries are described as deep cycle and have high charge and discharge cycles of at least 1500 cycles
These batteries are described as deep cycle and have high charge and discharge cycles of at least 1500 cycles
Unlike car batteries, which do not exceed 400 cycles at their best capabilities. The self-discharge of solar batteries is also low.
Types of solar batteries
The most popular types of deep cycle solar batteries are:
- Flooded lead acid batteries.
- AGM batteries.
- Gel batteries.
- Lithium-Ion batteries.
All of these deep cycle batteries can be used in solar power systems.
What are the most important reasons why we need deep cycle batteries instead of car batteries in solar energy systems?
- The charging and discharging current of deep cycle batteries is low.
- Longer charging and discharging time.
- Long life (more than 1500 charge and discharge cycles).
- Low self-discharge (i.e. does not empty when left for a long time).
Lifespan of solar batteries and car batteries
As shown in the curve when using 50% of the solar battery capacity on a daily basis,
The battery life is approximately 2000 charge and discharge cycles, while the car battery may give us 400 cycles when using 60% of the capacity.
Gel batteries
 Gel batteries
Gel batteries
Gel batteries and how to charge them,
Gel batteries are a type of battery that can be recharged for further use in powering DC loads or in a solar energy system.
It can be operated under a wide temperature range specified by the manufacturer, and it does not require regular maintenance.
In this important topic, we will talk about the most important information that may interest you about gel batteries and how to charge them in the best way, based on the manufacturer’s data.
What are gel batteries?
Gel batteries are known as one of the types of batteries that are specifically designed for deep cycle performance.
This makes it suitable for solar energy systems to operate electrical loads for long periods, depending on the amperage capacity of the battery.
Gel batteries store electrical energy coming from solar panels or the national grid inside the battery in the form of chemical energy during charging.
While the chemical energy inside the battery is converted into electrical energy in the form of direct current (DC) when connected to an electrical circuit or when operating an electrical device.
Gel battery
Gel battery components
Gel batteries consist of silica added to the electrolyte (a material that conducts electrical current) to form the gel material.
Containing a gel material makes it safer compared to liquid batteries.
It is also tightly sealed, making it impermeable to spilling on and off.
In addition to its high resistance to vibration and low internal resistance, which allows it to have excellent and high charging and discharging.
How to charge gel batteries
The process of charging gel batteries is done by connecting the two ends of the charger designated for this type of battery to the two ends of the battery, placing the positive with the positive and the negative with the negative.
Care must be taken that the charging voltage is approximately 14.2V~14.4 and the float voltage is between 13.5V~13.8V. These values are taken from the Ritar DG12-200 battery data sheet.
The discharge capacity of the battery should not exceed 80% to maintain it, and it is preferable, as much as possible, for the discharge rate to be within 50% of its capacity.
Battery electrodes
A table showing the charge percentage with battery voltage
| Shipping percentage | Open circuit voltage of the battery |
| 100% | 12.73 |
| 90% | 12.62 |
| 80% | 12.50 |
| 70% | 12.37 |
| 60% | 12.24 |
| 50% | 12.10 |
| 40% | 11.96 |
| 30% | 11.81 |
| 20% | 11.66 |
| 10% | 11.51 |
A table showing the charge percentage with battery voltage
Important note:
The charge percentage values compared to the battery voltage may differ from one battery to another, so it is preferable to read the data sheet for the electric battery.
Advantages of gel batteries
- The safest one.
- Protected from spills.
- Sealed tightly.
- Its high efficiency.
- Visit its virtual age.
- Excels in deep cycle.
- Its internal resistance is low.
- Designed for everyday use.
- Low self-discharge.
Disadvantages of gel batteries
- Its high cost.
- You need a special type of charger or charging regulator to ensure proper charging without causing damage to the battery.
Important note:
Although the cost of gel batteries increases to certain limits, they are preferable when considering the price over the period of use.
We will notice that gel batteries excel in the number of deep charge and discharge cycles.
You can use gel batteries in some electricity and solar energy systems with confidence and comfort because they are designed for deep cycle.
In addition, we must pay attention to the information about gel batteries and how to charge them with the appropriate charger recommended by the battery manufacturer.
What is an AGM battery?
 What is an AGM battery?
What is an AGM battery?
What is an AGM battery? The AGM battery is a type of rechargeable battery that is used in many different applications.
As it was manufactured with advanced advantages over the lead acid battery, what is an AGM battery and what are its advantages and disadvantages.
What is an AGM battery?
It is an abbreviation for “Absorbed Glass Mat” which means the absorbent glass mat battery. It is a battery designed with superior technologies compared to the lead-acid battery.
This battery was developed in 1985 AD for military fighter jets in order to reduce its weight and increase its capacity and stability.
The acid is absorbed by the ultra-soft fiberglass mat to make the battery spill-proof, enabling us to charge and transport it without any risks.
AGM battery
Uses of AGM battery
The AGM battery is generally used in many different applications, including:
It is used in luxury cars to operate accessories that require power, such as:
- Heaters, mirrors, steering wheels and windshield wipers are also used in racing cars because they are resistant to noise.
- They are used in marine, domestic engines and robotic applications due to their good performance at cold temperatures.
- Used in UPS units that require high power.
Advantages of AGM battery
- Its internal resistance is very low.
- Capable of giving high currents on demand.
- Its lifespan is relatively long even when cycled deeply.
- Doesn’t require maintenance.
- Offers good electrical capacity.
- Its weight is lighter than that of a lead acid battery.
- Works well at low temperatures.
- It has low self-discharge.
- Resistant to vibrations.
- They can be charged five times faster than liquid batteries.
- They have a longer cycle life than liquid batteries.
Cons of AGM battery
- Its cost is higher than that of a liquid battery.
- It is sensitive to overcharging.
- Its capacity gradually decreases with time.
- Its specific energy is low.
- Not environmentally friendly.
Final information:
The AGM battery is sensitive to overcharging, like all sealed and gel batteries, as each cell can be charged up to 2.4V.
Lithium batteries
 Lithium batteries
Lithium batteries
Lithium batteries: These batteries come under one name, namely lithium-ion batteries, but the internal chemical compounds may differ from one battery to another.
Therefore, when looking at lithium battery data, you notice symbols and numbers in the form of a chemical formula that differ from one battery to another depending on the manufacturer.
What are the types of lithium batteries, what compounds are found inside each type, and is there a difference between the types? We will talk about all of this here.
Contents
What is a lithium battery
Many beginners may think that there is only one type called lithium-ion battery,
But this belief is wrong because lithium batteries have many different types, differing mainly in cathode (positive electrode) materials.
There is also a difference in the anode (the negative electrode) by replacing it with a material such as graphite.
Scientists prefer to refer to batteries by their full name.
Chemical distinction, short formula and abbreviations. like:
Lithium-ion battery with the chemical compound LiCoO2, abbreviated LCO.

Types of lithium batteries
Lithium cobalt oxide (LiCoO2) – LCO.
Lithium manganese oxide (LiMn2O4) – LMO.
Lithium Nickel-Manganese Cobalt Oxide (LiNiMnCoO2) – NMC.
Lithium Iron Phosphate (LiFePO4) – LFP.
Nickel-Cobalt Aluminum Oxide (LiNiCoAlO2) – NCA Lithium Cobalt Oxide Battery
Lithium Cobalt Oxide Battery
(in English: Lithium Cobalt Oxide), material LiCoO2 (60%Co), abbreviation “LCO”, chemical formula “Li-cobalt”. “.
This type is characterized by high capacity, which makes it the best choice for mobile phones, laptops, and cameras. Its characteristics include:
Single cell voltage is 3.6 V (operating range is 3 V to 4.2 V).
Capacity is 150-200 watt-hours per kg.
Charging (C-rate): 0.7-1C Charging at 4.2V.
Discharging (C-rate): 2.50V-1C.
Number of charge and discharge cycles What is Between 500 to 1000 cycles (depending on depth of discharge and temperature).
Moderate operating temperature.
Lithium cobalt oxide battery
Lithium Manganese Oxide Battery
(in English: Lithium Manganese Oxide), material (LiMn2O4), abbreviation “LMO”,
Its chemical formula is “Li-manganese”.
It features low internal resistance, improved current handling and thermal stability.
However, its lifespan is less than that of lithium cobalt oxide battery.
The low internal resistance of the cell allows for fast charging and high-current discharging. The characteristics of the battery include:
Cell voltage 3.7V.
Typical operating range of battery voltage is 3 to 4.2V per cell.
Specific energy (capacity): 150- 100 watts/hour per kg.
The charging voltage is 4.2 volts, the maximum discharge voltage is 2.5 volts.
The number of charge and discharge cycles is between 300 and 700 cycles depending on the depth Discharging.
Moderate operating temperature.
Lithium manganese oxide battery
Lithium Nickel Manganese Cobalt Oxide Battery
(English: Lithium Nickel Manganese Cobalt Oxide)
, material LiNiMnCoO2 (10-20% Co), abbreviation “NMC”
Chemical formula “NMC”,
Lithium batteries are the most successful because they contain a cathode mixture of nickel, manganese and cobalt.
The components of this battery are characterized by low internal resistance, but they provide relatively low energy. Its characteristics include:
Cell voltage is 3.6 V (nominal: 3.7 V).
The operating voltage range is 3 to 4.2 V for the cell.
Specific energy (capacitance ) 150 to 220 watts/hour per kg.
The charging voltage is 4.2 volts and the discharging voltage is 2.5 volts.
The number of charging cycles is between 1000 to 2000 cycles depending on the depth of discharge And heat.
Lithium nickel manganese cobalt oxide battery
Lithium iron phosphate battery
(in English: Lithium Iron Phosphate), material (LifePO4), abbreviation “LFP”,
Chemical formula “Li-phosphate”.
Phosphate was discovered as a cathode material for lithium batteries in 1996 AD at the University of Texas and some contributors.
The material provides good electrochemical performance with low resistance and long life along with good thermal stability.
Among its characteristics:
Cell voltage is 3.2 V (nominal: 3.3 V).
The operating voltage range of the cell is 2.5 to 3.65 V.
Specific energy (capacitance) Ranging from 90 to 120 watts/hour per kg.
Moderate operating temperature.
The number of charge and discharge cycles reaches more than 2000 cycles.
Lithium iron phosphate battery
Nickel Cobalt Aluminum Oxide Battery
(English: Lithium Nickel Cobalt Aluminum Oxide), material (LiNiCoAIO2), abbreviation “NCA”, chemical formula “NCA”.
It has been used since 1999 in some special applications, and is characterized by good longevity, and the addition of aluminum gives it the most stable and stable chemical substance, and its characteristics include:
The nominal voltage of the cell is 3.6 volts.
The working voltage range of the cell ranges from 3 volts to 4.2 volts.
The specific power of the cell ranges from 200 to 260 watts. / hour per kg.
Charging voltage 4.20 V.
Deep discharge voltage 3.00 V.
Battery life 500 depending on depth of discharge and temperature .
Nickel Cobalt Aluminum Oxide Battery
Thus, we have mentioned to you the most popular types of lithium batteries supported by characteristics and illustrative pictures.
 Why is battery capacity measured in (Ah)?
Why is battery capacity measured in (Ah)?
 Why is battery capacity measured in (Ah)?
Why is battery capacity measured in (Ah)?
Why is the battery capacity measured in (Ah), when looking at the surface of a rechargeable battery such as a solar battery, for example,
You will notice its capacity in ampere-hours (Ah).
What is the reason behind classifying the battery in ampere-hours, and not watt-hours or kilowatt-hours?
So we have brought you detailed information about this question.
The importance of determining the battery capacity
Every electric battery has a specific capacity determined by the manufacturer in ampere-hours (Ah)
Depending on the nature of use and the extent of the effect of temperature on the internal cells of the battery and the percentage of discharge (DOD).
The battery capacity shows how much energy can be stored in the battery in the form of chemical energy
Which can be discharged as electrical energy in order to operate direct current (DC) loads.
However, the storage capacity of the battery may change by a slight increase or a sharp decrease depending on the number of charging and discharging cycles.
Or as the battery ages, or as a result of it being exposed to an inappropriate temperature.
That is, the battery capacity decreases more quickly over the years of use, as is the case with a smartphone battery.
Why is battery capacity measured in (Ah)
The main reason why manufacturers classify battery capacity in ampere-hours (Ah) and not watt-hours (Wh) or kilowatt-hours
(kWh) is the battery voltage, as we know that the battery voltage of the 12V system is variable, and ranges from 10.5V to 13.8V.
So in order to calculate the electrical energy stored in the battery,
You need to know two values, which are:
Amps and voltage. It is natural that the battery voltage decreases by a small amount, but the percentage of decrease depends on the age of the battery.
And it’s not just about ampere capacity,
But also other specifications, such as the battery discharge coefficient (C-rate), and the number of cycles (charging/discharging).
Also, the electrical energy that is discharged from the battery,
It is an amount of electrical charges that the battery pushes in order to feed electrical circuits.
These charges are called current, which are measured in ampere-hours.
Battery capacity in ampere-hours (Ah)
Meaning, if the battery has a specification of (12V/48Ah), this theoretically means that a fully charged battery can discharge 48 amps per hour.
But from the practical aspect of discharging the battery, there is an important parameter, which is the electric battery discharge coefficient
(C-Rate), that is, you cannot discharge the battery within just one hour, but there is a specific limit for discharge according to the value of (C-Rate).
Where the value of the parameter requires you to determine the current that must be discharged from the battery according to the number written next to the ampere capacity, and in most cases you write 10HR or 10C.
You may write with a higher value, such as 20HR.
The maximum discharge of the battery can be calculated by dividing the battery amperage capacity
(Ah) on the value of the discharge coefficient (C-Rate) in order to obtain the maximum ampere value that must be discharged per hour at
The total amperage capacity written on the surface of the battery in Ah.
The bottom line is that the capacity of large batteries is measured in ampere-hours in order to obtain the stored energy
By multiplying the total amperage capacity by the actual battery voltage value, this is because the battery voltage may decrease faster with time and the amount of load.
Different shapes of non-rechargeable batteries
That is, if you find a battery with a capacity of 10 ampere hours (10Ah) that gives a current of 10 amps for one hour, or it can give a current of 1 amp for 10 hours,
Thus, the battery capacity is equal to the product of amperes times hours.
When running loads on a battery, these loads must be estimated in watts or kilowatts.
The electrical energy consumed by these loads is given in watt-hours or kilowatt-hours.
It is the product of power multiplied by the operating duration in hours, and is written in the following formula:
Electrical energy consumed in watt-hours = power in watts x operating period in hours
Some people prefer to know the battery capacity in watt-hours instead of amperes, and this is easily done by multiplying the ampere-hour by the battery voltage.
Choose the required battery capacity
It is intended to operate five LEDs operating on 12 volt direct current for 8 hours continuously per day, the capacity of each LED
5 watts, calculate the battery capacity required to provide sufficient power, noting that the battery discharge rate should not exceed 50% during the operating period.
Total power consumed = power in watts x quantity (number) x number of operating hours
= 5 × 5 × 8
= 200 watt hours
Now we convert the value of watts to amps as follows:
Battery amperage capacity = power ÷ voltage
= 200 ÷ 12
= 16.6 ampere hours
New battery capacity at only 50% discharge (DOD = 50%):
New capacity = battery capacity x 2
= 16.6 × 2
= 33.2 amp hours
So you can choose any battery with an amperage capacity of no less than 33 amp hours.
Important note: The battery is available in the market with standard amperage capacities, that is, you cannot find a battery with a capacity of 33 amp hours, but there are 40 amp hours, and so on.
Choosing the appropriate battery capacity for loads (DC)
Choosing the appropriate battery capacity for loads (DC)
Choosing the appropriate battery capacity for loads (DC)
A very important topic for everyone who wants to buy a new battery so that it can operate direct current (DC) loads.
Some may think that an electrical load can run on any battery capacity without
Make any calculations that should have been taken into consideration before randomly selecting the battery.
In this topic, we will explain to you in a simplified manner how to choose the appropriate battery capacity for DC loads.
So that everyone can rely on the mathematical steps that we will mention in this article to facilitate the process of sizing the necessary battery capacity to suit the size of the direct current (DC) loads.
What is the battery capacity
Battery capacity is usually measured in amperes. Hour (Ah) (in English: Ampere. Hour)
That is, the battery with a storage capacity of 50 Ah gives a current of 50 amps for only one hour.
Or it can give a current of 10 amps for 5 hours, and so on. The summary of this is that the battery capacity is equal to the product of multiplying amps by hours.
However, the capacity of direct current (DC) loads is given in watts or kilowatts, while the electrical energy consumed by these loads is given in watt-hours or kilowatt-hours.
We can calculate the energy consumed in watts by multiplying the power value by the operating time in hours as follows:
Electrical energy consumed in watts. Hours = power in watts x number x operating period in hours
Battery capacity in watt-hours
Some may prefer to know the battery capacity in watt-hours instead of amperes. This is done by multiplying the ampere-hour by the battery voltage as follows:
Battery capacity in watt-hours = amps x volts
Example: 12 volt gel battery, storage capacity (100Ah). What is the battery capacity in watt-hours (W.h)?
Battery capacity in watt-hours = amps x volts
= 100 x 12 = 1200 watts. Hour(1200Wh)
Important note: You must choose a deep cycle battery intended for long-term operation and not a car battery.
Choose the appropriate battery capacity for the loads
Example:
There are 20 LEDs each with a capacity of 3W. All LEDs are intended to operate for 8 hours, and the load voltage is 12V/DC.
Calculate the power consumed by DC loads.
And choose the appropriate battery capacity for the loads?
the solution:
The energy it can consume from the battery = power in watts x number x operating period in hours
= 20 × 3 × 8
= 480 watts. hour (480Wh).
So when these loads are operated on a battery capacity for 8 hours, the power consumed from the battery is 480 watts. hour.
In order to preserve the battery for the longest possible period, and to obtain more cycles (charging and discharging), it is preferable to consume (50%) of the battery capacity as follows:
New storage energy = energy that can be consumed from the battery x 2
= 480 × 2
= 960 watts. hour (960Wh).
So we need a battery with a storage capacity of 960 watts. At least an hour, and the battery capacity can be converted from watts. Hours to ampere-hours are as follows:
Battery capacity (Amp-hours) = Storage energy (Watt-hours) ÷ Battery voltage
= 960 ÷ 12
= 80 amps. Hours (80Ah).
Therefore, we can choose a battery with the following specifications: (80Ah/12VDC).
Battery capacity 80Ah and voltage 12
We note that there is another condition that determines the safety of the load on the battery, which is the C-Rate discharge coefficient.
The lower the discharge coefficient value, the better.
Thus, we have explained to you a simplified method for choosing the appropriate battery capacity for the loads based on the electrical power law.
Using the same steps explained in the article, you can size the battery that suits your electrical loads.
The electric batteryand its types
 For more products and exclusive offers, please subscribe to our social media pages.
For more products and exclusive offers, please subscribe to our social media pages.
To view our Facebook page, click here.
To view our Twitter page, click here.









Leave a Reply
You must be logged in to post a comment.